As the U.S. prepares for nationwide distribution of vaccines to combat COVID-19, some are asking whether people who get the first of two doses will return to complete the series. The leading vaccine candidates from Pfizer/BioNTech and Moderna both require individuals to receive a second shot within a specific timeframe to achieve maximum effectiveness.
This analysis draws on Medicare Part D prescription drug claims data for the herpes zoster vaccine Shingrix, which also requires two doses, to shed light on this potential challenge of the leading COVID-19 vaccine candidates. Shingrix is recommended for adults ages 50 and older to prevent herpes zoster, also known as shingles, a viral infection that causes a painful rash and can lead to long-term pain and other problems. The second dose of Shingrix is to be administered between 2 and 6 months after the first dose. Overall, one-third of adults ages 60 and older in 2018 reported having ever received a shingles vaccine, but this estimate does not provide insight into which groups of older adults were more or less likely to get the second dose within the recommended timeframe after having received the first.
To address this question, we looked at Medicare beneficiaries who received an initial dose of Shingrix in the first half of 2018 to analyze what share received the second dose within the recommended timeframe and which subgroups of beneficiaries were more or less likely to receive both doses. Because people 65 and older are expected to be one of the earlier groups to receive COVID-19 vaccination, this analysis offers insight into what the experience might be among older adults in receiving the full regimen of multidose COVID-19 vaccines.
The majority of Medicare beneficiaries who received an initial dose of the Shingrix vaccine received the second dose within six months, but follow-up rates were lower among beneficiaries in communities of color, those who are younger than age 65 with long-term disabilities, and low-income beneficiaries.
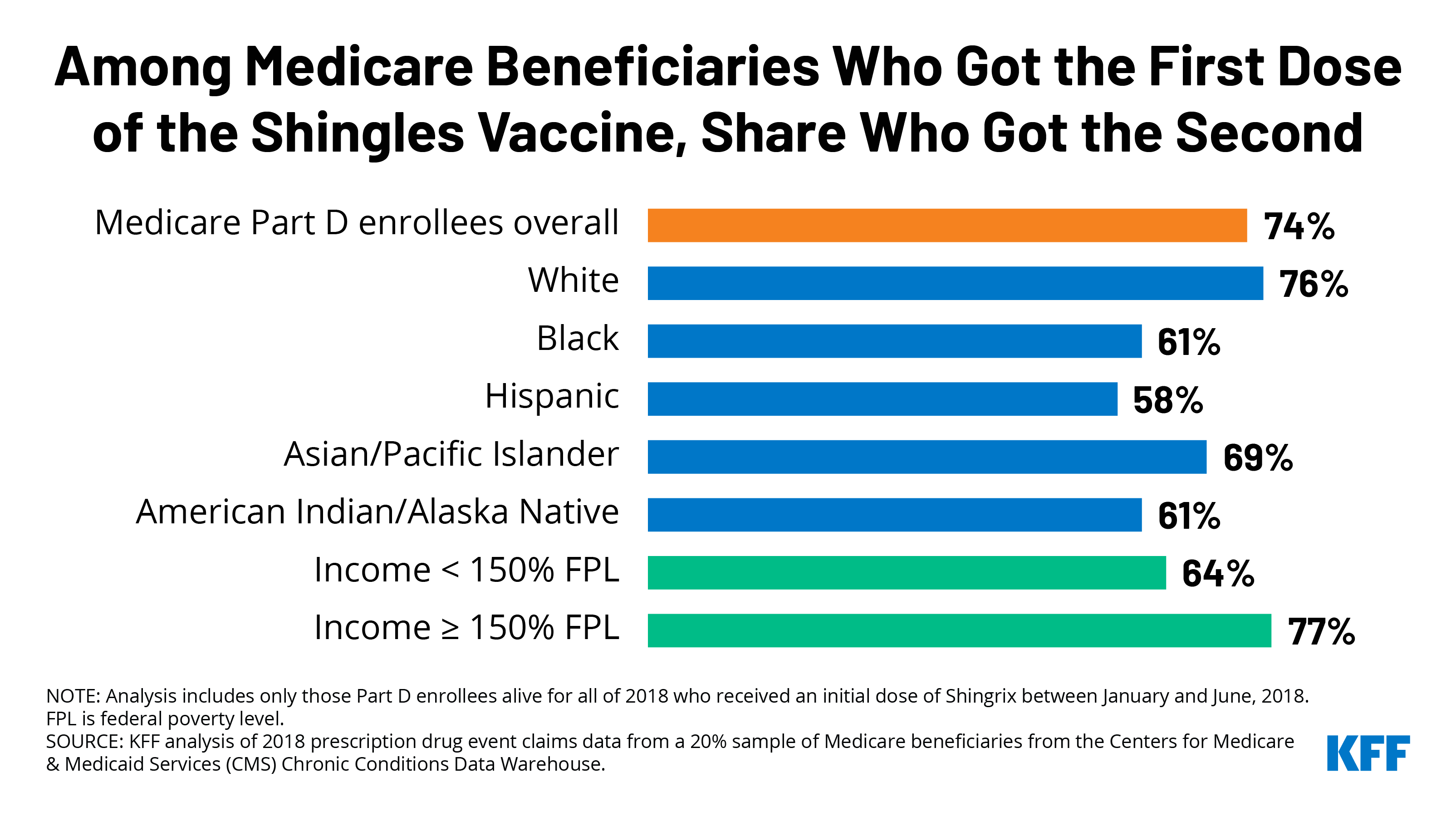
Most (74%) Medicare beneficiaries who received an initial dose of Shingrix between January and June of 2018 received the second dose within 6 months (Figure 1). Conversely, 1 in 4 beneficiaries (26%) who received an initial dose of Shingrix between January and June 2018 did not receive the second dose within the recommended timeframe. An additional 6% of beneficiaries received the second dose after the 6-month timeframe but no later than the end of 2018.
- Follow-up Shingrix vaccination rates were higher among White beneficiaries (76%) than among Hispanic (58%), American Indian/Alaska Native (61%), Black (61%), and Asian/Pacific Islander beneficiaries (69%). In other words, roughly 4 in 10 Black, Hispanic, and American Indian/Alaska Native beneficiaries did not receive their second shingles shot within the recommended 6-month timeframe. The share of beneficiaries receiving the second dose by the end of 2018 was higher among each group, but all estimates for beneficiaries of color were lower than for White beneficiaries.
- Medicare beneficiaries under age 65, who qualify for Medicare because of a long-term disability, were less likely than beneficiaries ages 65 and older to receive a second dose of Shingrix within 6 months. Among beneficiaries under age 65 who received a first dose of Shingrix between January and June of 2018, 66% received a second dose within 6 months of their first dose – a lower rate than among beneficiaries ages 65 to 74 (75%), 75 to 84 (76%), and 85 and older (71%).
- Beneficiaries with incomes less than 150% of poverty were less likely than beneficiaries with higher incomes to receive the second dose of the shingles vaccine within 6 months. (We used the share of beneficiaries receiving Part D low-income subsidies (LIS) as a proxy for low income). Only 64% of beneficiaries with lower incomes received the second dose within 6 months of their first dose in 2018, compared to 77% of those with higher incomes.
Notably, unlike the COVID vaccine which will be covered at no cost for Medicare beneficiaries, the Shingrix vaccine is not free to Medicare beneficiaries without LIS, but it is covered at very low cost to beneficiaries who receive LIS. In 2018, Medicare Part D enrollees without LIS paid an average of $57 out of pocket for each shot, while those who received LIS paid $5. (Under Part D, a separate copayment is required for each dose in the series.) It is possible that out-of-pocket costs deterred some beneficiaries from getting the follow-up shingles vaccine, but other factors may also be barriers to completing the series, such as lack of communication between providers and patients or misunderstanding about the necessity of the second dose, the hassle factor of a return visit to a doctor’s office or pharmacy for the second shot, or being deterred by adverse effects after the first dose. Patients can sign up on the Shingrix website to receive a second dose reminder, but doing so requires knowledge and action by patients. Research shows that pharmacist reminder calls can also help boost compliance with the shingles vaccine series, but this may not happen systematically across all providers.
The fact that the second dose of the two leading COVID-19 vaccine candidates is administered no more than one month after the first dose – versus up to 6 months between the first and second doses of the shingles vaccine – could mitigate some of the loss to follow up observed with the shingles vaccine. Moreover, preliminary evidence showing that the two COVID-19 vaccines closest to FDA authorization are highly effective in preventing COVID-19, a potentially fatal disease, may translate to higher take-up rates for the second shot than we observed with Shingrix. In addition, states and vaccine providers are being encouraged by the Centers for Disease Control and Prevention to attempt to schedule a second dose appointment at the time of a patient’s first dose. As part of a national vaccine education campaign, having systems in place for providers to communicate with patients about returning for a second dose is likely to be important in ensuring full compliance with the new COVID-19 vaccines. But the differences we observed in the percent of beneficiaries in different racial and ethnic groups, different age cohorts, and different income levels who received the second dose of Shingrix also underscore the challenges ahead in inoculating vulnerable populations against COVID-19.
Juliette Cubanski and Tricia Neuman are with KFF. Anthony Damico is an independent consultant.
| This analysis is based on 2018 Medicare Part D prescription drug event claims data from a 20% sample of Medicare beneficiaries from the Centers for Medicare & Medicaid Services (CMS) Chronic Conditions Data Warehouse (CCW). Our analysis includes 0.8 million Part D enrollees who were enrolled for the full 2018 calendar year and who received an initial shot of Shingrix between January and June of 2018. Shingrix was approved by the U.S. Food & Drug Administration in October 2017.
Our estimate of beneficiaries with incomes less than 150% of the federal poverty level (FPL) is based on the share of Part D enrollees receiving full or partial Part D Low-Income Subsidies (LIS). |
"who" - Google News
December 14, 2020 at 09:06PM
https://ift.tt/2IQg7kg
Who Didn't Get a Second Shingrix Shot? Implications for Multidose COVID-19 Vaccines - Kaiser Family Foundation
"who" - Google News
https://ift.tt/36dvnyn
https://ift.tt/35spnC7
Bagikan Berita Ini














0 Response to "Who Didn't Get a Second Shingrix Shot? Implications for Multidose COVID-19 Vaccines - Kaiser Family Foundation"
Post a Comment